The pH value is A measure of hydrogen ion concentration of the soil water system and expresses the acidity and alkalinity of soil. pH is very important property of soil as it determines the nutrient availability, microbial activity and physical condition of the soil.
The concept of pH was given by Sorensen in 1901. The pH of solution has been defined as negative logarithm of hydrogen ion activity which in very dilute solution is expressed as g ions L-1 or g mol L-1.
pH = – log10[H+] or [H+] = 10-ph
Itis found that in aqueous system at 220C
[H+][OH–] = 10-14
Which is called ionic product of water.
In soil water system, some of the adsorbed hydrogen ions dissociate from the surface of the soil colloids into soil solution. These dissociated H+ give rise to soluble acidity or active acidity. pH is a sort of voltage measurement and to cover the entire range of 0-14, a potential measurement in the range of + 420 to – 420 mV is needed. A potential difference of 59.1 mV is developed for a difference of one pH unit.
Instrument used for pH determination is a glass electrode pH meter with a calomel or reference electrode introducing salt bridge. Most digital pH meters now a days have a single or combined electrode assembly.
Apparatus required: pH meter, beaker (100 mL), measuring cylinder, glass rod and a balance.
Reagents: Buffers of different pH values (4.0, 7.0 and 9.2) for standardization or calibrating the pH meter. Dissolve one tablet in 100 mL of solution.
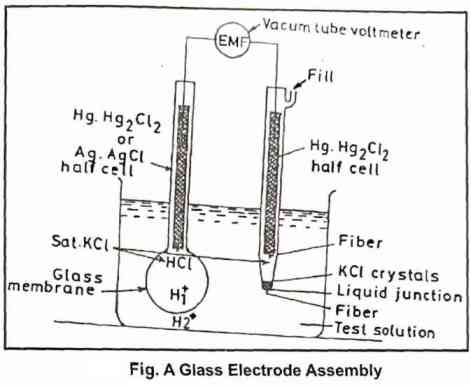
Description of pH meter
a) Glass electrode (indicator electrode)
This electrode is a glass membrane electrode consisting of hydrogen ion responsive thin-walled soft glass bulb sealed to a stem of non-hydrogen responsive high resistance glass. Both the inner and outer surfaces of the bulb are sensitive to the changes in H+ ion concentration. The inside bulb is filled with dilute HCl. When the glass electrode bulb is dipped in a solution having H+ concentration different from the inside electrode, an electrical potential develops across the membrane and this potential is proportional to the difference in pH between the two cells.
b) Calomel electrode (reference electrode)
It consists of Hg in contact with KCI saturated with Hg2Cl2, this electrode is having a constant potential. The two electrodes are connected to a potentiometer and a galvanometer which reads the pH.
Procedure
- Weigh 20 g of air-dried soil into a 100 mL beaker.
- To this add 50 mL of distilled water.
- Stir the contents with a glass rod intermittently for 30 minutes.
- Switch on the pH meter and allow for warming.
- Calibrate with the known buffers.
- Immerse the electrodes in soil water suspension and record the pH reading.
Interpretation of soil pH
| S. No. | pH range | Rating | Remarks |
| 1 | <4.5 | Very strongly acidic | Needs liming |
| 2 | 4.5-5.5 | Strongly acidic | Needs liming |
| 3 | 5.5-6.5 | Slightly acidic | Needs liming |
| 4 | 6.5-7.5 | Neutral | Suitable for crop growth |
| 5 | 7.5-8.5 | Slightly alkaline | — |
| 6 | 8.5-10.0 | Strongly alkaline | Needs gypsum application |
| 7 | >10.0 | Very strongly alkaline | Needs gypsum application |
Result: The pH of soil water suspension (1:2.5) is ——– and it is rated as ———-









